Our Innovations
BackBeat Cardiac Neuromodulation Therapy™, also known as atrioventricular interval modulation (AVIM) therapy, is designed to substantially, immediately and persistently lower blood pressure.
Programmable and adjustable bioelectronic therapy designed to lower blood pressure while simultaneously modulating the autonomic nervous system (ANS).

Designed to leverage standard rhythm management device hardware, such as dual-chamber pacemaker, utilizing the same implant procedure and lead positions.
Designed to immediately, substantially and persistently lower blood pressure.
Designed to substantially lower blood pressure through programmed pacing.
Designed to maintain reduction by modulating autonomic nervous system (ANS) response through programmed variable pressure patterns.
Designed to work automatically without relying on patient compliance with background antihypertension medications.
Designed to readily incorporate into existing devices and treatment pathways.
Can be readily incorporated into existing cardiac rhythm management devices such as pacemakers using standard implant and lead placement techniques.
Designed to be rapidly adaptable for hypertensive patients already indicated for a pacemaker.
Atrioventricular interval modulation (AVIM) therapy is supported by encouraging preclinical and clinical data. In MODERATO I and II pilot studies, the therapy demonstrated statistically significant reductions in blood pressure in patients with hypertension and an indication for a pacemaker.
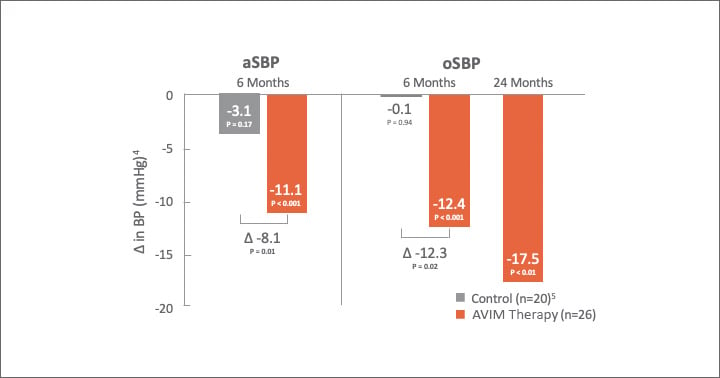
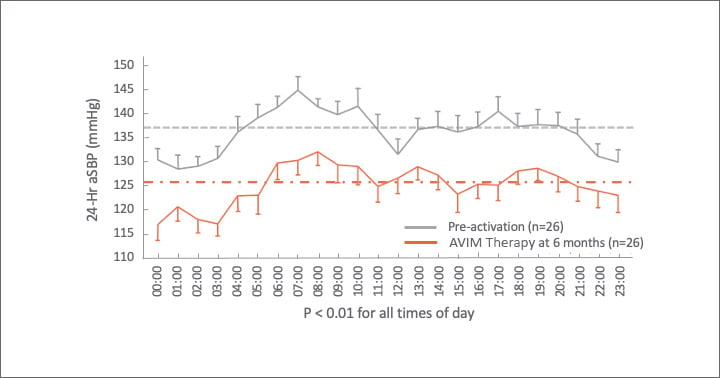
AVIM therapy showed promising results in MODERATO II, a prospective, multi-center, randomized, double-blind study of pacemaker patients with persistent hypertension including 88.5% with isolated systolic hypertension (ISH)1,2
mmHg 24-Hour aSBP3 at 6 months
mmHg in oSBP3 at 2 years
MACE3 at 6 months
of patients with reduction in aSBP
Significant Reduction in 24-Hr aSBP and oSBP
Results were presented during the Breakthrough Science Session at TCT 2019
Significant Reduction in aSBP 24 Hours a Day
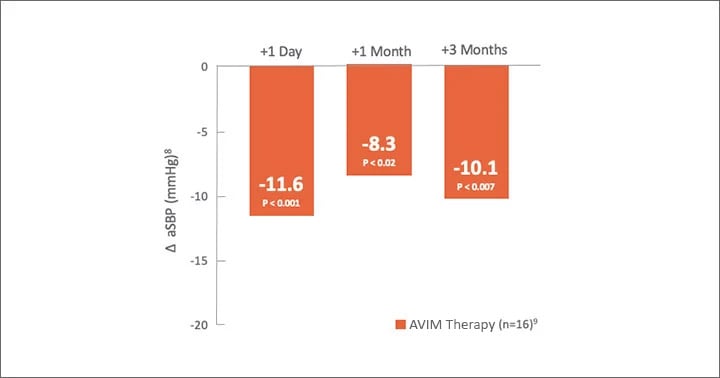
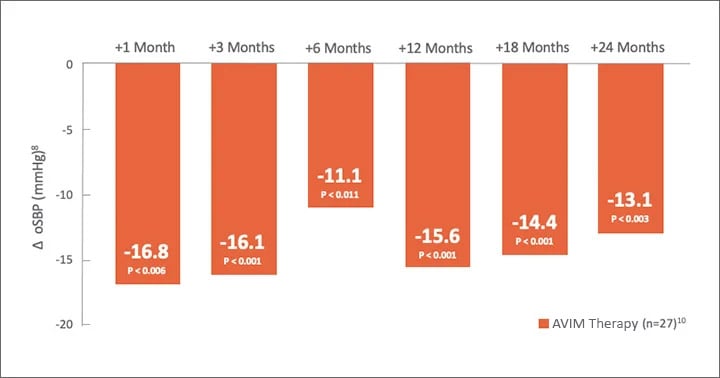
MODERATO I, an open-label, single-arm, multi-center, prospective study of 27 patients with persistent hypertension and an indication for a pacemaker demonstrated a substantial reduction in blood pressure sustained out to 2 years.6,7
mmHg 24-Hour aSBP at 3 months
mmHg in oSBP at 2 years
of patients with reduction in aSBP
Significant Reduction in 24-Hr aSBP
Significant Reduction in oSBP Maintained Through 24 Months
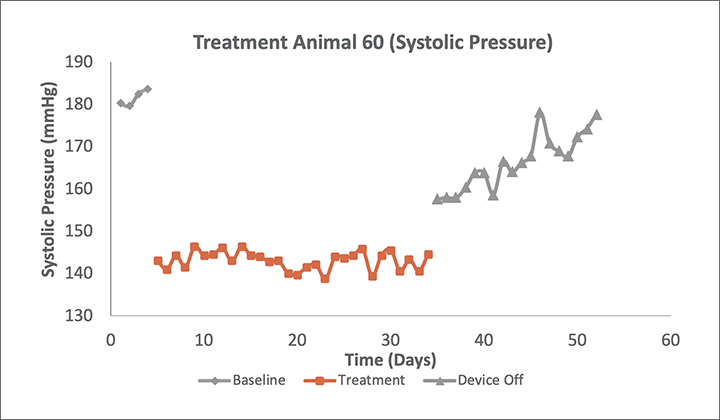
Preclinical Delivery of AVIM therapy significantly reduced 24-hour systolic and diastolic blood pressures in a canine model. The reduction occurred immediately upon activation of therapy and was maintained for its full duration.
Significantly Reduced Blood Pressure
*Data on file
1Kalaras et al. Journal of the American Heart Association. 2021;10:e020492. https://doi.org/10.1161/JAHA.120.020492.
2Burkhoff MODERATO II Study 2-Year Results TCT 2021.
3Ambulatory systolic blood pressure (aSBP), office systolic blood pressure (oSBP) and Major Cardiac Advance Events (MACE), including death, heart failure, clinically significant arrhythmias (i.e., persistent or increased atrial fibrillation, serious ventricular arrhythmias), myocardial infarction, stroke and renal failure in treatment group calculated per patient.
4Compared to pre-activation.
524-Hr aSBP Control (n=19). 1 control patient could not be measured despite repeat measurement (patient had extremely high blood pressure)
6Neuzil et al. Journal of the American Heart Association. 2017;6:e006974. https://doi.org/10.1161/JAHA.117.006974.
7Burkhoff MODERATO I Study 2-Year Results TCT 2018.
8Compared to pre-activation.
916 patients had aSBP at pre-activation.
1021 of 27 patients continued after completion of study at 3 months to be followed for 2 years
Orchestra BioMed entered into a global strategic collaboration with Medtronic to develop AVIM therapy as a bioelectronic treatment for hypertension in patients indicated for a cardiac pacemaker.


"As the global leader in advanced cardiac pacing therapies, Medtronic is the ideal partner to help us develop AVIM therapy for the treatment of hypertension, which is remarkably common and drives significant health risk in the pacemaker population. This exemplifies our commitment to accelerating high-impact medical innovations with global medical technology leaders."
David Hochman,
Chairman, CEO and Founder, Orchestra BioMed
About the Agreement
BackBeat CNT™ (also known as AVIM therapy) is investigational and not commercially approved.
© 2025 Orchestra BioMed Inc. Virtue®, BackBeat CNT™ FreeHold Duo®, FreeHold Trio® and Orchestra BioMed™ are trademarks of Orchestra BioMed.
All other trademarks are trademarks of their respective owners.
SM-0020 Rev 01