Our investigational technologies, AVIM Therapy and Virtue SAB, have the potential to transform transform clinical outcomes and provide distinct commercial advantages within two of the largest and most well-established global medical device markets.
Hypertension ("HTN"), or elevated blood pressure, is the leading global risk factor for death, affecting 1.2B+ patients worldwide. It increases the risk of severe cardiovascular events such as heart attack, stroke, heart failure and kidney disease. (source: World Health Organization)
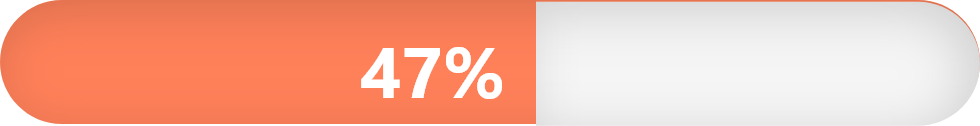

Number and percentage of adults estimated to have HTN in the United States (source: have HTN according to the American Heart Association):
Cardiovascular risk doubles for every 10 mmHg increase in systolic blood pressure (source: NCBI).
Mortality rate doubles with an increase of 20 mmHg in systolic blood pressure (source: NCBI).
According to new ACC/AHA guidelines, nearly 80% of U.S. patients indicated for a pacemaker have hypertension. Over 60% of these patients have uncontrolled hypertension, putting them at higher risk for Isolated Systolic Hypertension, which is harder to treat. Pacemaker patients average 73 years old and often have other conditions like atherosclerosis, hyperlipidemia, diabetes, and chronic kidney disease. These patients could greatly benefit from hypertension treatments such as AVIM therapy, administered through their pacemakers.
Artery disease, caused by plaque buildup in the arteries (atherosclerosis), is the leading cause of death worldwide (source: WHO)
The most common treatment is percutaneous catheter-based intervention, including balloon angioplasty and stents. Procedures performed globally in 2019 (LSI & HRI Research):
Performed worldwide in 2019
Performed worldwide in 2019
We believe significant unmet needs remain in certain artery disease indications where treatment options are limited or fail to adequately improve patient outcomes, including coronary in-stent restenosis, coronary small vessel disease, lesions in patients with high bleeding risk and below-the-knee peripheral vascular disease.
Virtue® SAB is investigational and not commercially approved.
AVIM Therapy is investigational and not commercially approved.